Precision Medicine
Systemic Mastocytosis
Systemic mastocytosis (SM) is a rare, clonal mast cell neoplasm driven by the KIT D816V mutation1,2
Patients experience significant symptom burden with poor quality of life that can result in a reduced ability to work, higher rates of health care visits, use of multiple medications, and severe pain3
Activated mast cells release granules containing proinflammatory mediators and can result in:
- Maculopapular lesions with Darier’s sign
- Recurrent or unexplained anaphylaxis often coupled with hypotension and syncope
- Anaphylaxis due to insect sting
- Recurring and unexplained gastrointestinal upset (i.e., recurring and unexplained nausea, vomiting, and/or diarrhea)
- Persistently elevated baseline serum tryptase levels
- Serum tryptase levels that increase by 20% above the baseline level plus an additional 2 ng/mL if measured within 4 hours after the onset of the acute event3
- Unexplained osteoporosis (particularly in males)
- Unexplained hepatopathy with ascites
- Presence of adult onset cutaneous mastocytosis
- Chronic use of prescription medications for the treatment of unexplained allergies (e.g., corticosteroids, mast cell stabilizers)
Proceed to a diagnostic workup if SM is suspected with symptoms consistent with mast cell disorder and no known cause:
- A diagnostic workup consisting of a history and physical including metabolic panel, CBC with differential, and blood smear examination
- Test for serum tryptase levels
- Molecular testing for KIT D816V; NCCN Guidelines recommends a highly sensitive assay such as ASO-qPCR or digital droplet PCR on peripheral blood for initial screening; A thorough analysis of KIT mutational status should include bone marrow evaluation
- Bone marrow aspirate and biopsy with flow cytometry (CD34, CD117, CD25, CD30, CD2), IHC (CD117, CD25, CD30, tryptase), cytogenetics
- FISH as needed for associated hematologic neoplasm (AHN)-related abnormalities
- Test for additional genomic mutations via NGS, especially in advanced SM
- Evaluation of B- and C- findings and organ involvement
Media
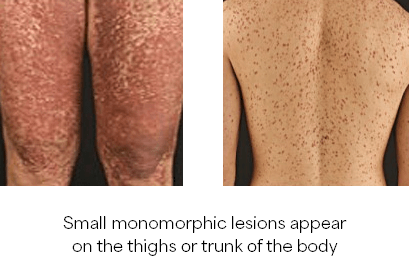
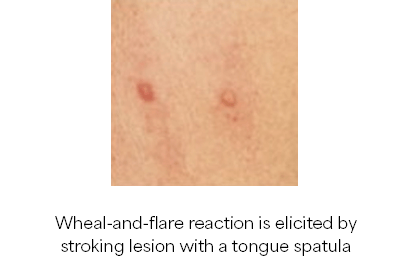
Diagnosis of SM requires the major criterion alone (ICC)10 or with 1 minor criteria (WHO)12 OR ≥3 minor criteria (ICC and WHO)10, 12, 13:
Major Criterion
- Multifocal dense infiltrates of MCs (≥15 mast cells in aggregates) detected in sections of bone marrow and/or sections of other extracutaneous organ(s) such as the GI tract
Minor Criterion
- More than 25% of MCs in bone marrow (biopsy section or aspirate smears) or other extracutaneous organ(s) show abnormal morphology (i.e., atypical MC type 1 or are spindle-shaped MCs) in multifocal lesions in histologic examination
- KIT mutation at codon 816 in extracutaneous organ(s) (in most cases bone marrow) or peripheral blood
- KIT+ MCs in bone marrow show abnormal expression of CD25 (and/or less specifically CD2)
- Serum total tryptase > 20 ng/mL (except in patients with associated hematologic neoplasm (AHN)-type disease.)
If SM is confirmed, proceed to identification of SM subtype11, 12:
- Indolent SM
- Smoldering SM
- Aggressive SM
- SM with an associated hematologic neoplasm
- Mast Cell Leukemia
Indolent SM
Meets the general criteria for systemic mastocytosis; <2 B-findings*; No C-findings¥; Low mast cell burden; No evidence of an associated hematologic neoplasm; Skin lesions are frequently present.
Smoldering SM
Meets the general criteria for systemic mastocytosis; ≥2 B-findings; No C-findings; No evidence of an associated hematologic neoplasm; Does not meet the criteria for mast cell leukemia.
Aggressive SM
Meets the general criteria for systemic mastocytosis; ≥1 C-finding; Does not meet the criteria for mast cell leukemia; Skin lesions are usually absent.
SM with an associated hematologic neoplasm
Meets the general criteria for systemic mastocytosis; Meets the criteria for an associated neoplasm.
Mast Cell Leukemia
Bone marrow aspirate smears show ≥20% mast cells; In classic cases, mast cells account.
*B-Findings: Indicate a high burden of MCs and expansion of the neoplastic process into multiple hematopoietic lineages, without evidence of organ damage
¥C-Findings: Are indicative of organ damage produced by MC infiltration (should be confirmed by biopsy if possible)
Indolent & Smoldering SM
- Referral to specialized centers
- Patient education
- Avoiding triggers
- Use of epinephrine to manage anaphylaxis
- Anti-mediator drug therapy
- Clinical trial
- Avapritinib for ISM
Agressive SM
- ISM and SSM treatment options
- Clinical trial
- Avapritinib (if platelets ≥50 x 109/L) or midostaurin
- Cladribine or peginterferon alfa-2a ± prednisone
SM with an associated hematologic neoplasm
- ISM and SSM treatment options
- Avapritinib (if platelets ≥50 x 109/L) or midostaurin
- Other recommended regimens: cladribine or peginterferon alfa- 2a ± prednisone
- Immedate treatment requirement or on progression: AHN-directed therapy including consideration of allogenic HCT with concurrent management of SM
Mast Cell Leukeima
- ISM and SSM treatment options
- Avapritinib or midostaurin
- Other recommended regimens: cladribine
- AHN-directed therapy (including multiagent chemotherapy)
- Consider evaluation for allogeneic HCT
Media
High Sensitivity cKIT D816V Mutation Hotspot

Connect with
Dr. Pankit Vachhani
Submit a question about Systemic Mastocytosis and Dr. Vachanni will respond.
References:
